Polymer fractionation

Separation principle
Liquid/liquid phase separation
The fractionation of polymers is somewhat more difficult than for low molecular weight mate-rials. The typical separation methods like distillation, fractional crystallization or liquid-liquid extraction fail for polymers as their volatility is too low and many of them do not crystallize. The liquid-liquid extraction by means of incompletely miscible solvents is not applicable as the dif-ference in solubility of molecules with dissimilar chain lengths is by far too small. The whole material would be found in one phase only. In almost all cases it is mandatory to perform the separation of macromolecules in the dissolved state. A very powerful method is the preparative GPC which enables the access to different fractions in a single experiment. However, the amount of polymer that is fractionated in this manner does normally not exceed 1 g of polymer and scale-up for the production of several kg or tons per day is impractical or at least uneconomic. In order to fractionate large amounts ultrafiltration can be used. However, this technique suffers from sev-eral fundamental drawbacks: Once a certain membrane is chosen, the molar mass at which the cut through the molecular weight distribution takes place is fixed. Further disadvantages are the fouling tendency of the membranes and the considerable amounts of solvent required due to the fact that the separation is normally performed with dilute polymer solutions.
Large scale fractionation techniques that avoid these drawbacks are based on liquid-liquid phase separation. The figure shows how such a phase separation can be achieved for a binary sys-tem consisting of a polydisperse polymer and one solvent. The solvent power is reduced in such a manner, that an initially homogenous polymer solution (called feed, FD) demixes into a polymer rich phase (gel, GL) and a polymer lean phase (sol, SL). This can be achieved either through a change in temperature (route A) or in composition (route B) by adding pure solvent (extracting agent, EA) to the feed solution. In both cases the condition of the working point (WP) is reached which lies within the miscibility gap (shaded area).
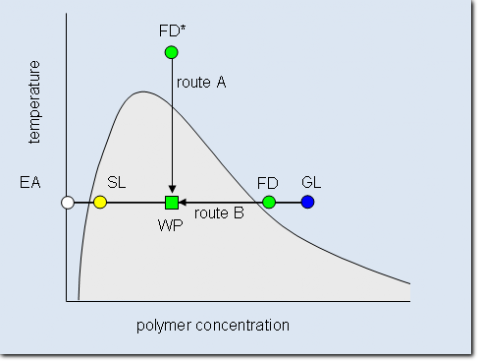
Schematic phase diagram of a binary system consisting of polymer and solvent. The miscibility gap below the cloud point curve is shaded. Indicated in the graph is how the compositions of the working point (WP) is reached either by cooling down a homogenous feed solution (FD*, route A) or by adding the pure solvent (Extracting agent, EA) to a concentrated feed solution (FD, route B). The system demixes into a polymer rich gel phase (GL) and a polymer lean sol phase (SL).
In daily life binary systems are normally not used for fractionation because the miscibility gap is for a given polymer determined by the choice of the solvent. In many cases the fractionation would have to be performed at unsuitable temperatures or concentrations. Therefore ternary sys-tems consisting of the polymer, a solvent, and a non-solvent are normally used for practical rea-sons. The following figure shows a typical ternary phase diagram of such a system in form of the so called Gibbs phase triangle. It gives the whole composition range of a ternary mixture at a constant temperature. The pure components are located in the corners while the binary subsys-tems are given by the edges. Ternary mixtures are represented by points lying inside the triangle. The schematic phase diagram depicted in the figure shows a typical representative as used for fractionation. The polymer and the solvent are at the given temperature miscible for the entire range of composition. Same is true for the mixtures of solvent and non-solvent while polymer and non-solvent exhibit a miscibility gap.
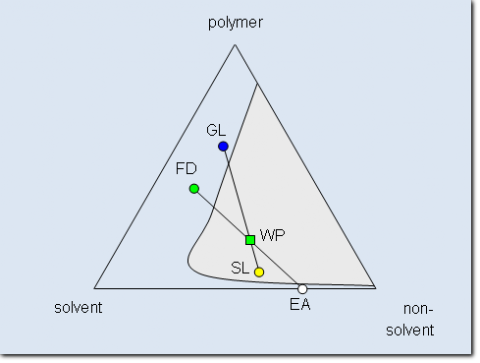
Schematic phase diagram of a ternary system consisting of polymer, solvent and non-solvent at constant temperature. The miscibility gap is shaded again. The composition of the working point is reached by adding the extracting agent (in this case consisting of solvent and non-solvent) to the feed solution.
Despite the increased complexity the ternary system has the advantage that normally for every polymer a suitable solvent/non-solvent system can be found to enable to operate at convenient temperatures. As indicated in the above figure the feed can be a ternary mixture and the extract-ing agent can consist of solvent and non-solvent. The working point lies inside the miscibility gap and therefore the mixture separates into the sol and gel phase.
The phase separation leads to an enrichment of the long chain material contained in an initially broadly distributed polymer in the gel phase because of enthalpic reasons, while the low molecu-lar weight material accumulates in the sol phase due to entropic reasons. As a result of this frac-tionation the compositions of the gel and of the sol phase do not lie on the cloud point curve of the starting material.
The following page shows how these basics are successfully applied to fractionated polymers by means of the "Continuous spin fractionation" (CSF):
