Absorption spectra - Homepage WEE-Solve GmbH
Main menu:
- Home
- Menütrennlinie 7
- About us
- Menütrennlinie 1
- Fractionation
- Menütrennlinie 4
- Rheology
- Menütrennlinie 5
- Contract research
- Menütrennlinie 6
- Analysis
- Menütrennlinie 8
- Contact
Absorption spectra
During spectroscopic measurements the molecules of the investigated sample were irradiated with electromagnetic waves of ultraviolet (UV; ca. 200 -
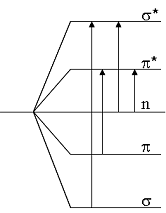
Molecular orbitals and electron transfers
The electron excitation is always coupled with a whole slew of possible vibration and rotation excitations. Because the measurement is performed in solution, the single absorptions become indistinct into a mutual curve, which is detected as a broad band in the spectrum.
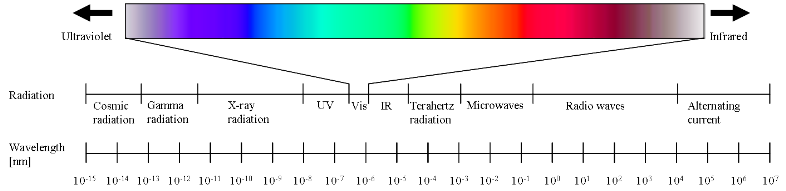
Radiation types and their wavelength range
The absorption spectroscopy can be established for qualitative as well as for quantitative analysis: Due to the form and the position of the absorption bands qualitative conclusions about the molecule can be made, which can e.g. be used for structure determination. A strong absorption can be traced back to the existence of specific structure elements in the molecule (so-
The table shows some chromophoric groups and their absorption peaks:
Chromophore |
Absorption peak |
Carbonyl group (ketones) RR'C=O |
271 nm |
Carbonyl group (aldehydes) RHC=O |
293 nm |
Carboxyl group RCOOH |
204 nm |
Amido group RCONH2 |
208 nm |
Azo group - |
339 nm |
Nitro group - |
280 nm |
The exact wavelengths of the absorption bands depend in addition to the substituent of the compound on the solvent respectively of the polarity of the solvent. An increasing solvent polarity shifts the absorption bands to lower wavelengths (so-
The position and the intensity of the absorption peaks are however additionally dependent on other parameters such as pH-
Some functional groups are here listed, which can be identified due to absorption spectroscopy:
Unsaturated rings and C-
C Multiple bonds Condensed aromatic hydrocarbons and unsaturated heterocyclic and homocyclic compounds
Conjugation and cumulation
Analysis of equilibria:
Protolytic equilibria
Complex formation equilibria
Charge-
Transition metal complexes
For the quantitative analysis the very high molar extinction coefficients are responsible for the sensitive detection limit of the UV/Vis-
Some possible determinations are:
Determination of elements by means of chelating agents (e.g. Dithizone method)
Determination of anions and ammonia by means of colour agents
Photometric water analysis of cations and anions by means of colour agents
Determination of organic compounds, above all conjugated systems