Separation principle - Homepage WEE-Solve GmbH
Main menu:
- Home
- Menütrennlinie 7
- About us
- Menütrennlinie 1
- Fractionation
- Menütrennlinie 4
- Rheology
- Menütrennlinie 5
- Contract research
- Menütrennlinie 6
- Analysis
- Menütrennlinie 8
- Contact
Separation principle
The fractionation of polymers is somewhat more difficult than for low molecular weight mate-
Large scale fractionation techniques that avoid these drawbacks are based on liquid-
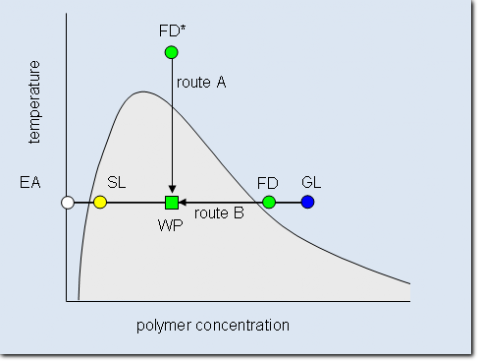
Schematic phase diagram of a binary system consisting of polymer and solvent. The miscibility gap below the cloud point curve is shaded. Indicated in the graph is how the compositions of the working point (WP) is reached either by cooling down a homogenous feed solution (FD*, route A) or by adding the pure solvent (Extracting agent, EA) to a concentrated feed solution (FD, route B). The system demixes into a polymer rich gel phase (GL) and a polymer lean sol phase (SL).
In daily life binary systems are normally not used for fractionation because the miscibility gap is for a given polymer determined by the choice of the solvent. In many cases the fractionation would have to be performed at unsuitable temperatures or concentrations. Therefore ternary sys-
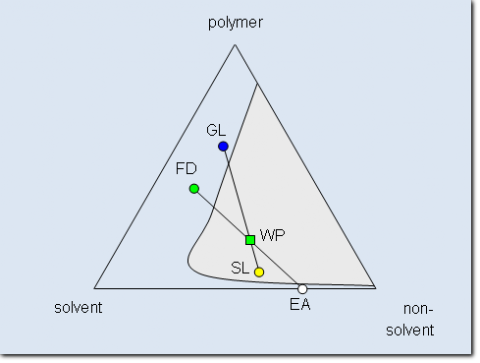
Schematic phase diagram of a ternary system consisting of polymer, solvent and non-
Despite the increased complexity the ternary system has the advantage that normally for every polymer a suitable solvent/non-
The phase separation leads to an enrichment of the long chain material contained in an initially broadly distributed polymer in the gel phase because of enthalpic reasons, while the low molecu-
The following page shows how these basics are successfully applied to fractionated polymers by means of the "Continuous spin fractionation" (CSF):
